Multiclass Diffential Expression Analysis
Multi-class / Multi-group Differential Expression Analysis
Introduction
In transcriptomics analysis, we are often interested in identifying differentially expressed genes. For example, if we have two conditions or cell types, we may be interested in what genes are significantly upregulated in condition A vs. B or cell type X vs. Y and vice versa. However, what happens when you have multiple conditions or many cell types? In this tutorial, I will use simulated data to demonstrate potential stategies for performing multi-class or multi-group differential expression analysis.
Simulation
First, let’s simulate 5 different classes/groups of samples (say, bulk RNA-seq of 5 different sorted cell types), each with 30 samples each, each expressing 1000 genes total. Note, in this very simplistic demonstration, we will assume that our gene expression follow a normal distribution.
set.seed(0)
G <- 5
N <- 30
M <- 1000
initmean <- 5
initvar <- 10
mat <- matrix(rnorm(N*M*G, initmean, initvar), M, N*G)
rownames(mat) <- paste0('gene', 1:M)
colnames(mat) <- paste0('cell', 1:(N*G))
group <- factor(sapply(1:G, function(x) rep(paste0('group', x), N)))
names(group) <- colnames(mat)
heatmap(mat, Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)
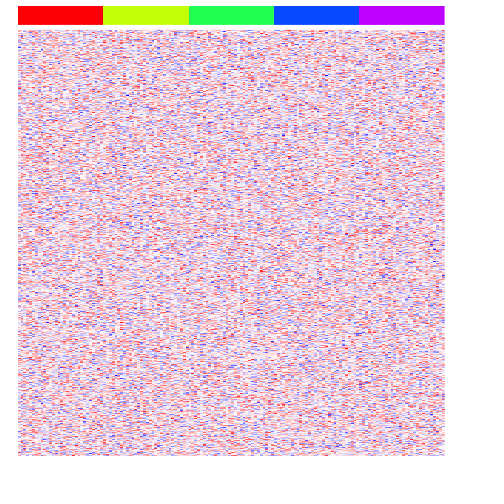
Now, let’s simulate some differentially upregulated genes unique to each group.
set.seed(0)
upreg <- 5
upregvar <- 10
ng <- 100
diff <- lapply(1:G, function(x) {
diff <- rownames(mat)[(((x-1)*ng)+1):(((x-1)*ng)+ng)]
mat[diff, group==paste0('group', x)] <<- mat[diff, group==paste0('group', x)] + rnorm(ng, upreg, upregvar)
return(diff)
})
names(diff) <- paste0('group', 1:G)
heatmap(mat, Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)
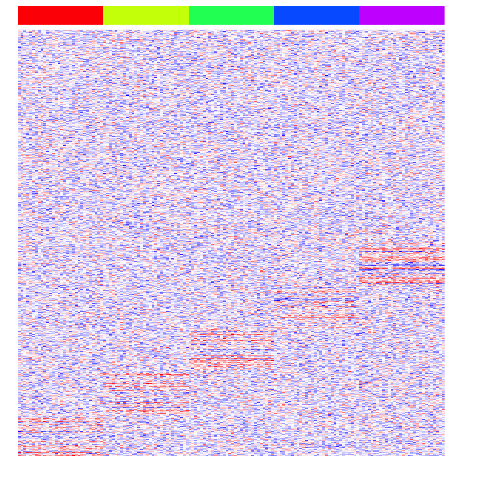
Let’s also simulate some differentially upregulated genes affecting groups of groups. This is typical of cell differentiation processes.
diff2 <- lapply(2:(G-1), function(x) {
y <- x+G
diff <- rownames(mat)[(((y-1)*ng)+1):(((y-1)*ng)+ng)]
mat[diff, group %in% paste0("group", 1:x)] <<- mat[diff, group %in% paste0("group", 1:x)] + rnorm(ng, upreg, upregvar)
return(diff)
})
heatmap(mat, Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)
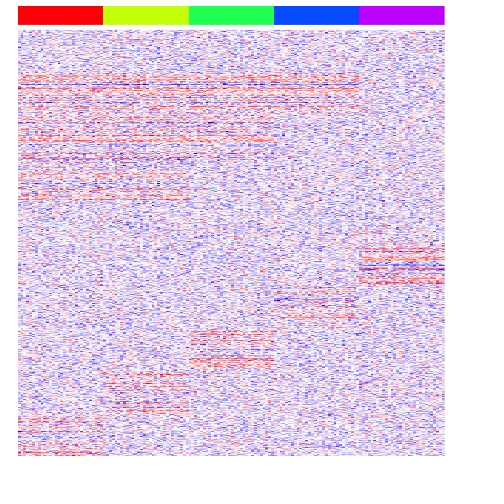
Now, we can begin testing different approaches for multi-group differential expression analysis.
1 vs many (in group vs. not in group)
One approach is to consider each group vs. all others. We can use a simple T-test to test whether each gene is significantly upregulated in each group vs. all other groups.
pv.sig <- lapply(levels(group), function(g){
ingroup <- names(group)[group %in% g]
outgroup <- names(group)[!(group %in% g)]
pv <- sapply(1:M, function(i) {
t.test(mat[i,ingroup], mat[i,outgroup], alternative='greater')$p.value
#t.test(mat[i,ingroup], mat[i,outgroup])$p.value
})
names(pv) <- rownames(mat)
pv.sig <- names(pv)[pv < 0.05/M/G] ## bonferonni
pv.sig
})
heatmap(mat[unique(unlist(pv.sig)),], Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)

Indeed, we pick up many of the differentially upregulated genes we simulated that are unique to each group ie. in diff. However, we have a much harder time picking up the differentially upregulated genes marking multiple groups, ie. in diff2 as expected.
perf <- function(pv.sig) {
predtrue <- unique(unlist(pv.sig)) ## predicted differentially expressed genes
predfalse <- setdiff(rownames(mat), predtrue) ## predicted not differentially expressed genes
true <- c(unlist(diff), unlist(diff2)) ## true differentially expressed genes
false <- setdiff(rownames(mat), true) ## true not differentially expressed genes
TP <- sum(predtrue %in% true)
TN <- sum(predfalse %in% false)
FP <- sum(predtrue %in% false)
FN <- sum(predfalse %in% true)
sens <- TP/(TP+FN)
spec <- TN/(TN+FP)
prec <- TP/(TP+FP)
fdr <- FP/(TP+FP)
acc <- (TP+TN)/(TP+FP+FN+TN)
return(data.frame(sens, spec, prec, fdr, acc))
}
print(perf(pv.sig))
## sens spec prec fdr acc
## 1 0.25625 1 1 0 0.405
ANOVA
Alternatively, we can use ANOVA (analysis of variance) to identify genes that are variable among and between groups.
pv <- sapply(1:M, function(i) {
mydataframe <- data.frame(y=mat[i,], ig=group)
fit <- aov(y ~ ig, data=mydataframe)
summary(fit)[[1]][["Pr(>F)"]][1]
})
names(pv) <- rownames(mat)
pv.sig <- names(pv)[pv < 0.05/M/G] ## bonferonni
heatmap(mat[pv.sig,], Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)
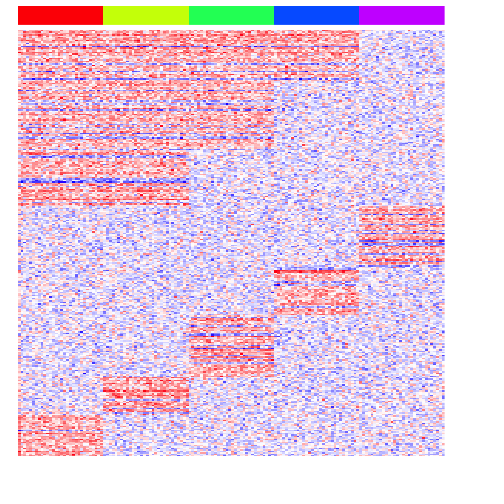
Note with ANOVA, we need to do an additional step to then figure out which group the variable gene is marking. But compared to testing each group vs. all others, we do get more genes that are differentially upregulated in multiple groups.
print(perf(pv.sig))
## sens spec prec fdr acc
## 1 0.3625 1 1 0 0.49
Testing combined groups (pairs, triplets, etc)
What if we test all possible pairwise combinations of groups?
pv.sig.pair <- lapply(levels(group), function(g1) {
g2 <- setdiff(levels(group), g1)
unlist(lapply(g2, function(g) {
## test two groups vs. all others
ingroup <- names(group)[group %in% c(g1, g)]
outgroup <- names(group)[!(group %in% c(g1, g))]
pv <- sapply(1:M, function(i) {
t.test(mat[i,ingroup], mat[i,outgroup], alternative='greater')$p.value
})
names(pv) <- rownames(mat)
pv.sig <- names(pv)[pv < 0.05/M/G] ## bonferonni
pv.sig
}))
})
heatmap(mat[unique(unlist(pv.sig.pair)),], Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)
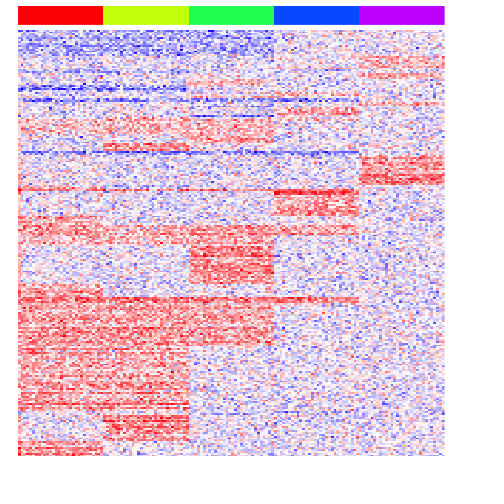
As expected, we now readily recover the genes upregulated in two groups.
print(perf(pv.sig.pair))
## sens spec prec fdr acc
## 1 0.28125 1 1 0 0.425
Of course, this approach becomes unreasonable if we have many groups. And if we are doing all pairs, why not all triplets. The number of combinations grow exponentially and quickly becomes intractable.
pv.sig.trip <- lapply(levels(group), function(g1) {
g2 <- setdiff(levels(group), g1)
unlist(lapply(g2, function(gi) {
g3 <- setdiff(levels(group), gi)
unlist(lapply(g3, function(gj) {
## test three groups vs. all others
ingroup <- names(group)[group %in% c(g1, gi, gj)]
outgroup <- names(group)[!(group %in% c(g1, gi, gj))]
pv <- sapply(1:M, function(i) {
t.test(mat[i,ingroup], mat[i,outgroup], alternative='greater')$p.value
})
names(pv) <- rownames(mat)
pv.sig <- names(pv)[pv < 0.05/M/G] ## bonferonni
pv.sig
}))
}))
})
heatmap(mat[unique(unlist(pv.sig.trip)),], Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)
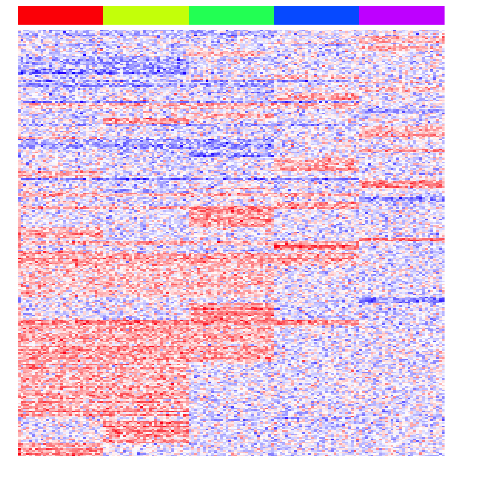
print(perf(pv.sig.trip))
## sens spec prec fdr acc
## 1 0.32625 0.995 0.9961832 0.003816794 0.46
Tree-based binary split
My preferred approach is to assume an underlying tree structure relating groups and compare groups based on recursive binary splits along the tree.
## average gene expression for each group
mat.group <- do.call(cbind, lapply(levels(group), function(g) {
rowMeans(mat[,group==g])
}))
colnames(mat.group) <- levels(group)
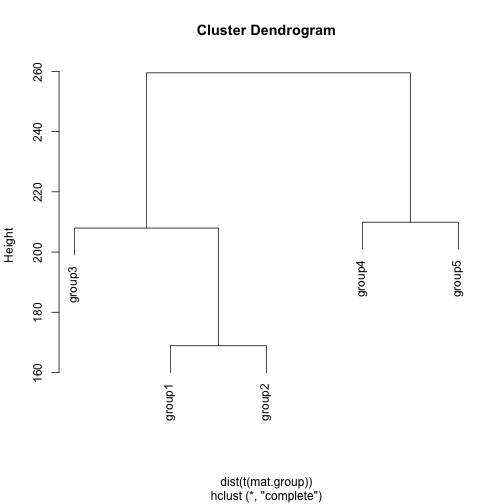
## construct tree related groups
hc <- hclust(dist(t(mat.group)), method='complete')
plot(hc)
dend <- as.dendrogram(hc)
pv.sig.all <- c()
pv.recur <- function(dend) {
g1 <- labels(dend[[1]])
g2 <- labels(dend[[2]])
#print(g1)
#print(g2)
ingroup <- names(group)[group %in% g1]
outgroup <- names(group)[group %in% g2]
pv <- sapply(1:M, function(i) {
t.test(mat[i,ingroup], mat[i,outgroup])$p.value
})
names(pv) <- rownames(mat)
pv.sig <- names(pv)[pv < 0.05/M/length(hc$height)] ## bonferonni
#print(pv.sig)
pv.sig.all <<- c(pv.sig.all, pv.sig) ## save
## recursion to go down tree if not leaf
if(!is.leaf(dend[[1]])) {
pv.recur(dend[[1]])
}
if(!is.leaf(dend[[2]])) {
pv.recur(dend[[2]])
}
}
pv.recur(dend)
heatmap(mat[unique(unlist(pv.sig.all)),], Rowv=NA, Colv=NA, col=colorRampPalette(c('blue', 'white', 'red'))(100), scale="none", ColSideColors=rainbow(G)[group], labCol=FALSE, labRow=FALSE)
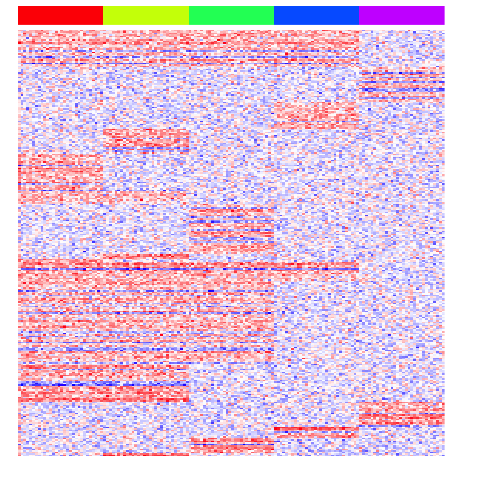
Of course, the performance of this approach will depend on the initial tree reconstruction.
print(perf(pv.sig.all))
## sens spec prec fdr acc
## 1 0.34 1 1 0 0.472
Conclusion
Try it yourself! What happens if we reduce the degree of upregulation upreg in the simulation? Or increase in variance? What if instead of using a normal, we simulate a zero-inflated negative binomial more characteristic of single cell RNA-seq data? What if we add in different levels of noise to each sample, differential library sizes, and so forth?
Recent Posts
- Vibe coding with SEraster and STcompare to compare spatial transcriptomics technologies on 22 February 2026
- RNA velocity in situ infers gene expression dynamics using spatial transcriptomics data on 13 October 2025
- Analyzing ICE Arrest Data - Part 2 on 27 September 2025
- Analyzing ICE Detention Data from 2021 to 2025 on 10 July 2025
- Multi-sample Integrative Analysis of Spatial Transcriptomics Data using Sketching and Harmony in Seurat on 22 April 2025