Aligning two coronal sections of adult mouse brain from MERFISH
In this notebook, we align two single cell resolution spatial transcriptomics datasets of coronal sections of the adult mouse brain from matched locations with respect to bregma assayed by MERFISH.
We will use STalign to achieve this alignment. We will first load the relevant code libraries.
[1]:
## import dependencies
import numpy as np
import matplotlib.pyplot as plt
import pandas as pd
import torch
import plotly
import requests
# make plots bigger
plt.rcParams["figure.figsize"] = (12,10)
[ ]:
# OPTION A: import STalign after pip or pipenv install
from STalign import STalign
[2]:
## OPTION B: skip cell if installed STalign with pip or pipenv
import sys
sys.path.append("../../STalign")
## import STalign from upper directory
import STalign
We have already downloaded single cell spatial transcriptomics datasets and placed the files in a folder called merfish_data.
We can read in the cell information for the first dataset using pandas as pd.
[3]:
# Single cell data 1
# read in data
fname = '../merfish_data/datasets_mouse_brain_map_BrainReceptorShowcase_Slice2_Replicate3_cell_metadata_S2R3.csv.gz'
df1 = pd.read_csv(fname)
print(df1.head())
Unnamed: 0 fov volume center_x \
0 158338042824236264719696604356349910479 33 532.778772 617.916619
1 260594727341160372355976405428092853003 33 1004.430016 596.808018
2 307643940700812339199503248604719950662 33 1267.183208 578.880018
3 30863303465976316429997331474071348973 33 1403.401822 572.616017
4 313162718584097621688679244357302162401 33 507.949497 608.364018
center_y min_x max_x min_y max_y
0 2666.520010 614.725219 621.108019 2657.545209 2675.494810
1 2763.450012 589.669218 603.946818 2757.013212 2769.886812
2 2748.978012 570.877217 586.882818 2740.489211 2757.466812
3 2766.690012 564.937217 580.294818 2756.581212 2776.798812
4 2687.418010 603.061218 613.666818 2682.493210 2692.342810
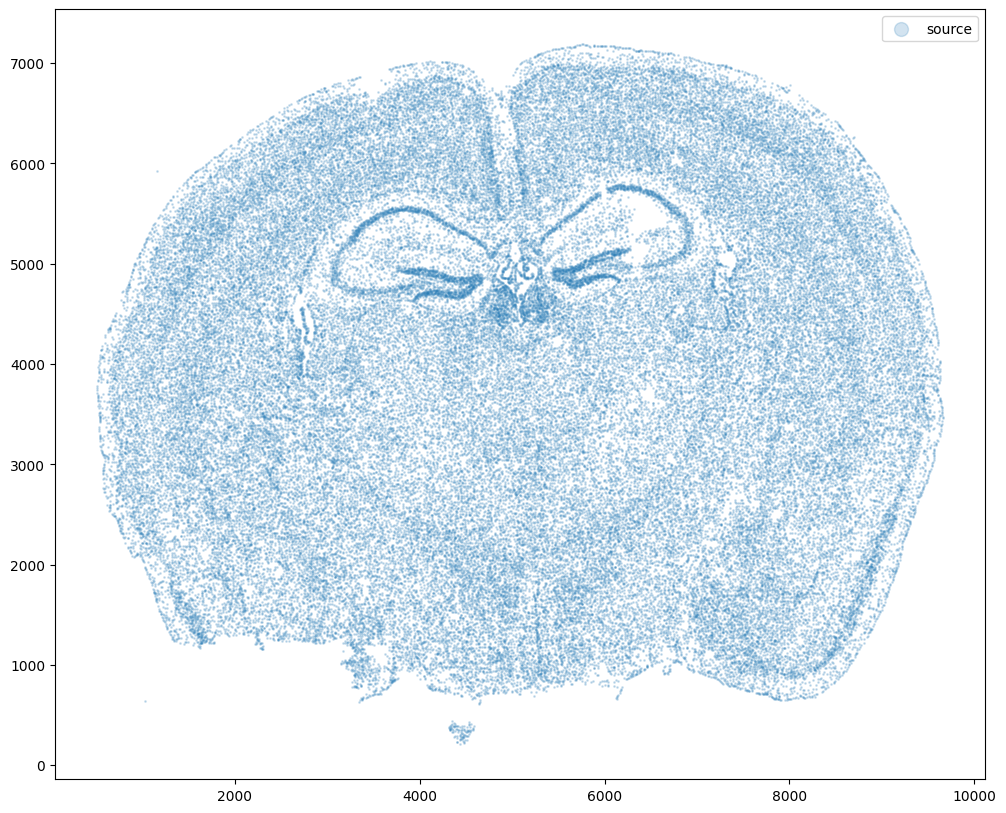
For alignment with STalign, we only need the cell centroid information so we can pull out this information. We can further visualize the cell centroids to get a sense of the variation in cell density that we will be relying on for our alignment by plotting using matplotlib.pyplot as plt.
[4]:
# get cell centroid coordinates
xI = np.array(df1['center_x'])
yI = np.array(df1['center_y'])
# plot
fig,ax = plt.subplots()
ax.scatter(xI,yI,s=1,alpha=0.2, label='source')
ax.legend(markerscale = 10)
[4]:
<matplotlib.legend.Legend at 0x7fce6fc43c40>
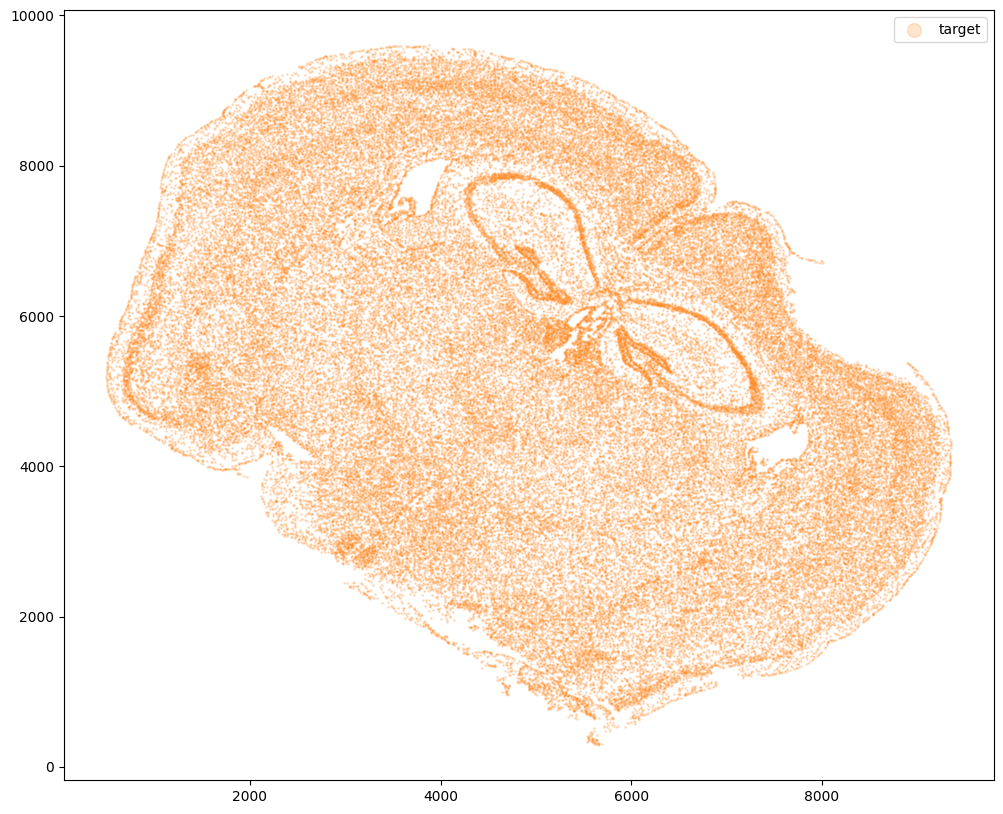
Now, we can repeat this to get cell information from the second dataset.
[5]:
# Single cell data 2
# read in data
fname = '../merfish_data/datasets_mouse_brain_map_BrainReceptorShowcase_Slice2_Replicate2_cell_metadata_S2R2.csv.gz'
df2 = pd.read_csv(fname)
# get cell centroids
xJ = np.array(df2['center_x'])
yJ = np.array(df2['center_y'])
# plot
fig,ax = plt.subplots()
ax.scatter(xJ,yJ,s=1,alpha=0.2,c='#ff7f0e', label='target')
ax.legend(markerscale = 10)
[5]:
<matplotlib.legend.Legend at 0x7fce6c78cfd0>
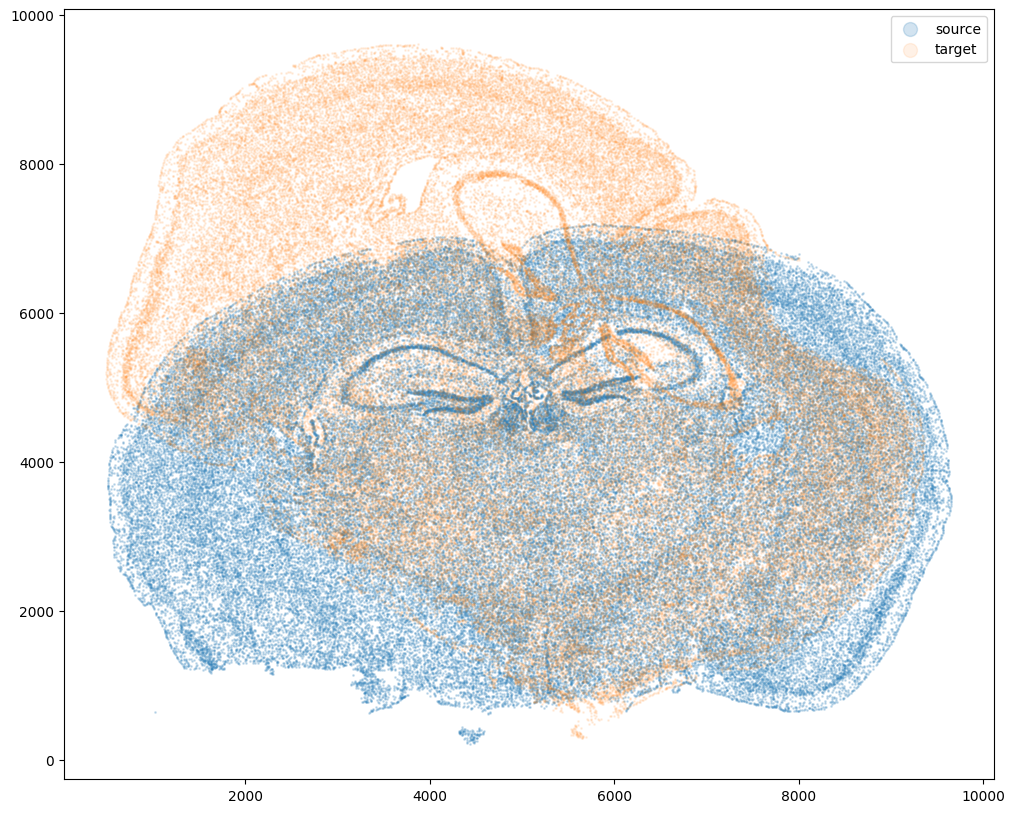
Note that plotting the cell centroid positions from both datasets shows that non-linear local alignment is needed.
[6]:
# plot
fig,ax = plt.subplots()
ax.scatter(xI,yI,s=1,alpha=0.2, label='source')
ax.scatter(xJ,yJ,s=1,alpha=0.1, label= 'target')
ax.legend(markerscale = 10)
[6]:
<matplotlib.legend.Legend at 0x7fce6c86afd0>
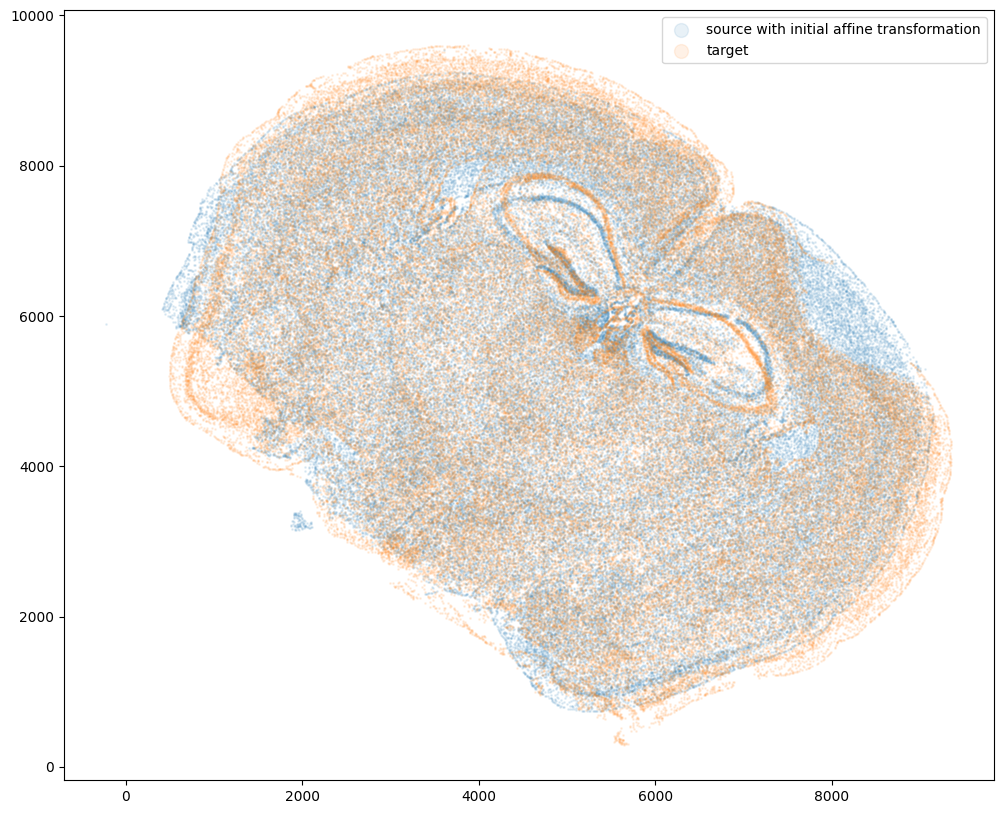
STalign relies on an interative gradient descent to align these two images. This performs quicker and better if the source and target are initially at a similar angle.
Evaluate the similarity of the rotation angle by viewing a side by side comparison. Change the value of theta_deg, until the rotation angle is similar. Note: the rotation here is defined in degrees and is in the clockwise direction.
The angle chosen will be used to construct a 2x2 rotation matrix L and a 2 element translation vector T.
[7]:
theta_deg = 45
theta0 = (np.pi/180)*-theta_deg
#rotation matrix
#rotates about the origin
L = np.array([[np.cos(theta0),-np.sin(theta0)],
[np.sin(theta0),np.cos(theta0)]])
source_L = np.matmul(L , np.array([xI, yI]))
xI_L = source_L[0]
yI_L = source_L[1]
#translation matrix
#effectively makes the rotation about the centroid of I (i.e the means of xI and yI])
#and also moves the centroid of I to the centroid of J
T = np.array([ np.mean(xI)- np.cos(theta0)*np.mean(xI) +np.sin(theta0)*np.mean(yI) - (np.mean(xI)-np.mean(xJ)),
np.mean(yI)- np.sin(theta0)*np.mean(xI) -np.cos(theta0)*np.mean(yI) - (np.mean(yI)-np.mean(yJ))])
xI_L_T = xI_L + T[0]
yI_L_T = yI_L + T[1]
fig,ax = plt.subplots()
ax.scatter(xI_L_T,yI_L_T,s=1,alpha=0.1, label='source with initial affine transformation')
ax.scatter(xJ,yJ,s=1,alpha=0.1, label = 'target')
ax.legend(markerscale = 10)
[7]:
<matplotlib.legend.Legend at 0x7fce6c8a35e0>
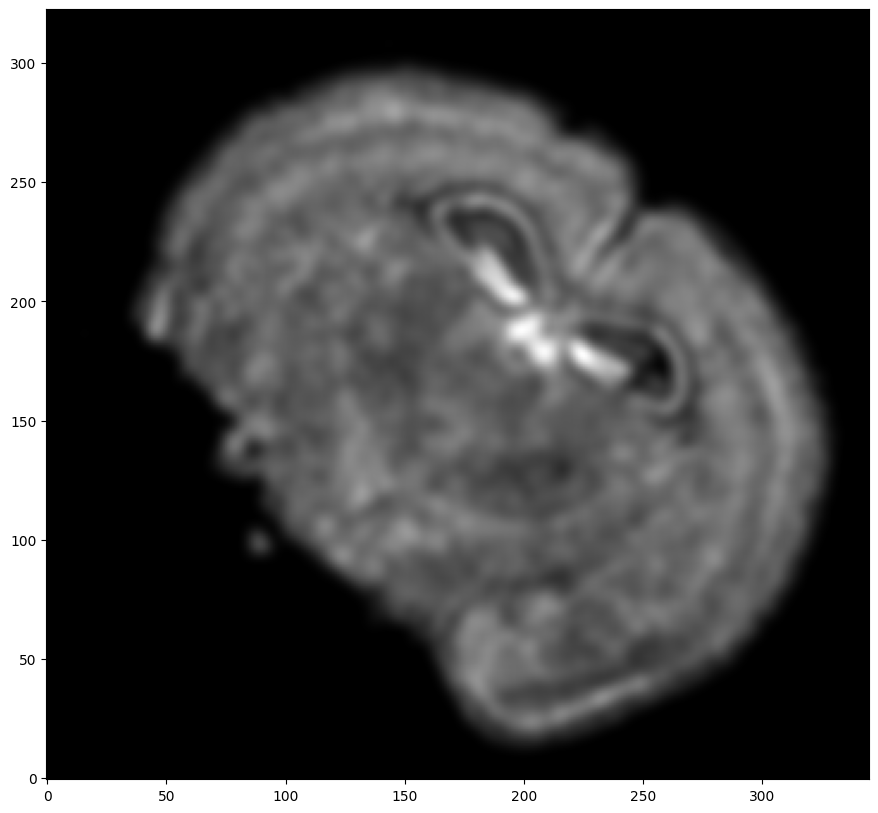
Now, we will first use STalign to rasterize the single cell centroid positions into an image. Assuming the single-cell centroid coordinates are in microns, we will perform this rasterization at a 30 micron resolution. We can visualize the resulting rasterized image.
Note that points are plotting with the origin at bottom left while images are typically plotted with origin at top left so we’ve used invert_yaxis() to invert the yaxis for visualization consistency.
[8]:
# rasterize at 30um resolution (assuming positions are in um units) and plot
XI,YI,I,fig = STalign.rasterize(xI_L_T,yI_L_T,dx=30,blur=1.5)
# plot
ax = fig.axes[0]
ax.invert_yaxis()
0 of 85958
10000 of 85958
20000 of 85958
30000 of 85958
40000 of 85958
50000 of 85958
60000 of 85958
70000 of 85958
80000 of 85958
85957 of 85958
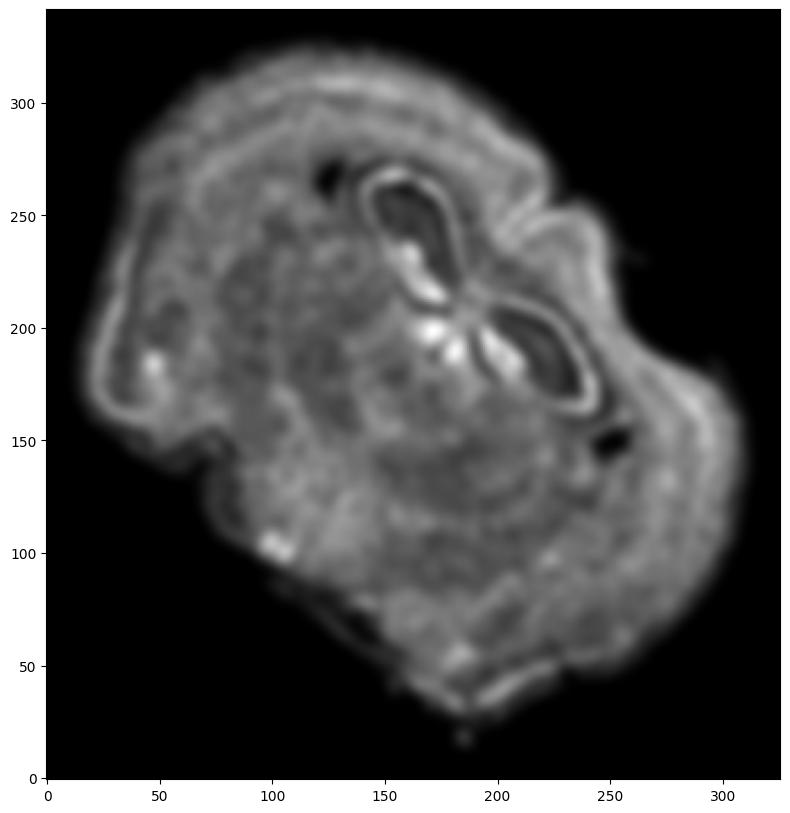
Repeat rasterization for target dataset.
[9]:
# rasterize and plot
XJ,YJ,J,fig = STalign.rasterize(xJ,yJ,dx=30, blur=1.5)
ax = fig.axes[0]
ax.invert_yaxis()
0 of 84172
10000 of 84172
20000 of 84172
30000 of 84172
40000 of 84172
50000 of 84172
60000 of 84172
70000 of 84172
80000 of 84172
84171 of 84172
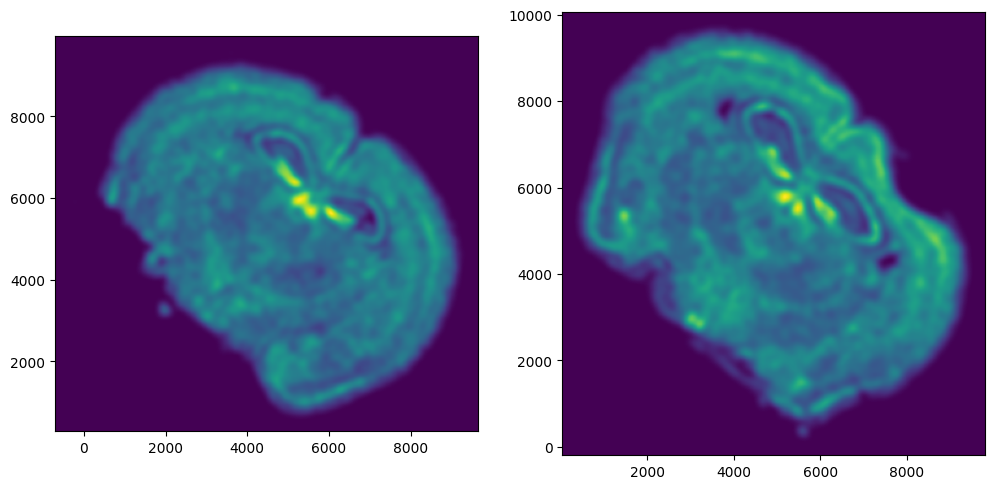
We can also plot the rasterized images next to each other.
[10]:
# get extent of images
extentI = STalign.extent_from_x((YI,XI))
extentJ = STalign.extent_from_x((YJ,XJ))
# plot rasterized images
fig,ax = plt.subplots(1,2)
ax[0].imshow(I[0], extent=extentI)
ax[1].imshow(J[0], extent=extentJ)
ax[0].invert_yaxis()
ax[1].invert_yaxis()
Now we will perform our alignment. There are many parameters that can be tuned for performing this alignment. If we don’t specify parameters, defaults will be used.
[11]:
%%time
# run LDDMM
# specify device (default device for STalign.LDDMM is cpu)
if torch.cuda.is_available():
device = 'cuda:0'
else:
device = 'cpu'
# keep all other parameters default
params = {
'niter': 10000,
'device':device,
'epV': 50
}
out = STalign.LDDMM([YI,XI],I,[YJ,XJ],J,**params)
/home/kalen/STalign/docs/notebooks/../../STalign/STalign.py:1043: UserWarning: To copy construct from a tensor, it is recommended to use sourceTensor.clone().detach() or sourceTensor.clone().detach().requires_grad_(True), rather than torch.tensor(sourceTensor).
L = torch.tensor(L,device=device,dtype=dtype,requires_grad=True)
/home/kalen/STalign/docs/notebooks/../../STalign/STalign.py:1044: UserWarning: To copy construct from a tensor, it is recommended to use sourceTensor.clone().detach() or sourceTensor.clone().detach().requires_grad_(True), rather than torch.tensor(sourceTensor).
T = torch.tensor(T,device=device,dtype=dtype,requires_grad=True)
/home/kalen/.local/share/virtualenvs/STalign-VWNsoi3D/lib/python3.8/site-packages/torch/functional.py:504: UserWarning: torch.meshgrid: in an upcoming release, it will be required to pass the indexing argument. (Triggered internally at ../aten/src/ATen/native/TensorShape.cpp:3483.)
return _VF.meshgrid(tensors, **kwargs) # type: ignore[attr-defined]
/home/kalen/STalign/docs/notebooks/../../STalign/STalign.py:1301: UserWarning: Data has no positive values, and therefore cannot be log-scaled.
axE[2].set_yscale('log')
CPU times: user 25min 25s, sys: 9.46 s, total: 25min 34s
Wall time: 14min 26s
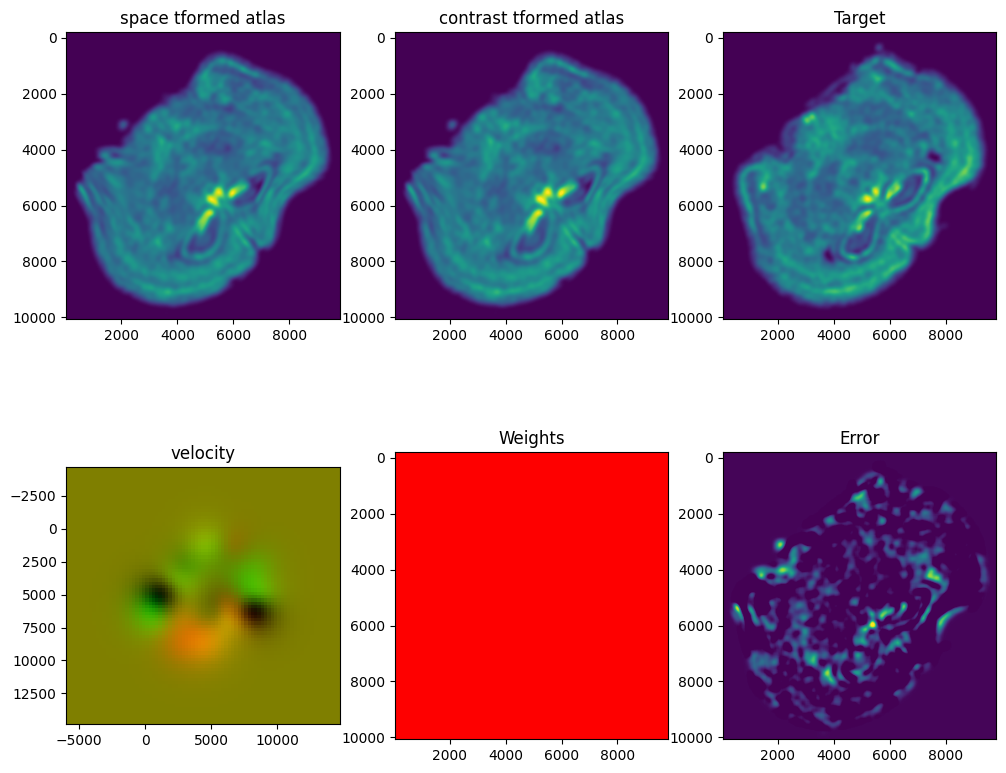
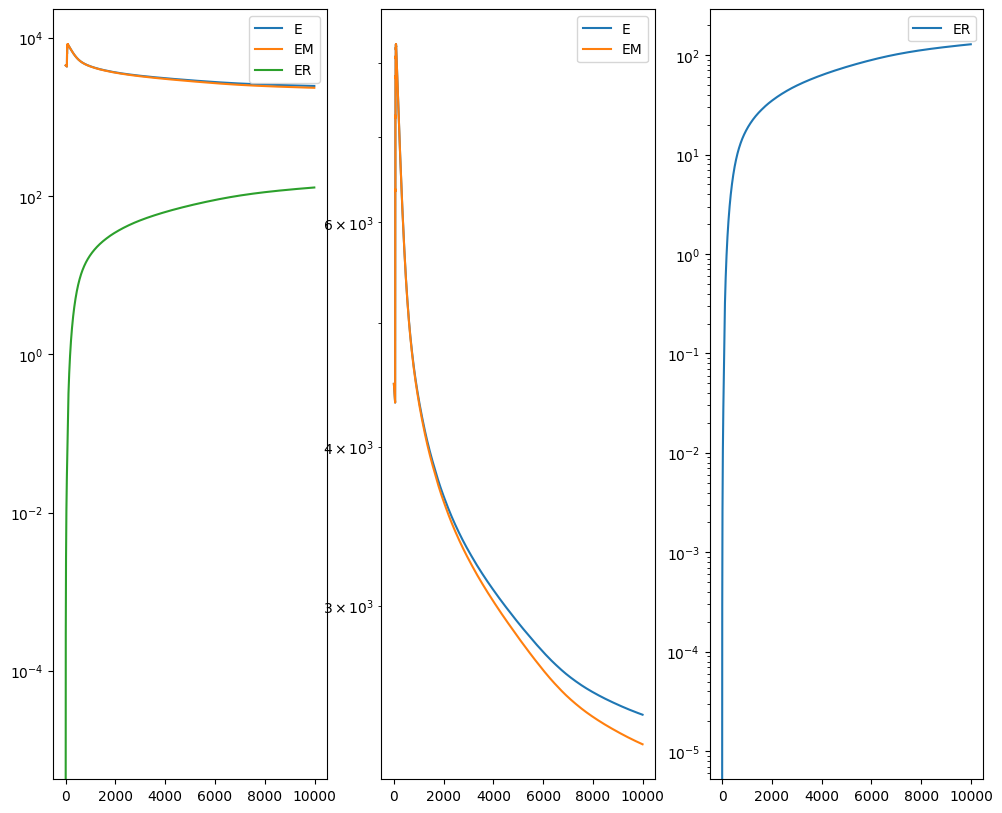
[29]:
# get necessary output variables
A = out['A']
v = out['v']
xv = out['xv']
Plots generated throughout the alignment can be used to give you a sense of whether the parameter choices are appropriate and whether your alignment is converging on a solution.
We can also evaluate the resulting alignment by applying the transformation to visualize how our source and target images were deformed to achieve the alignment.
[30]:
# set device for building tensors
if torch.cuda.is_available():
torch.set_default_device('cuda:0')
else:
torch.set_default_device('cpu')
[41]:
# apply transform
phii = STalign.build_transform(xv,v,A,XJ=[YJ,XJ],direction='b')
phiI = STalign.transform_image_source_to_target(xv,v,A,[YI,XI],I,[YJ,XJ])
#switch tensor from cuda to cpu for plotting with numpy
if phii.is_cuda:
phii = phii.cpu()
if phiI.is_cuda:
phiI = phiI.cpu()
# plot with grids
fig,ax = plt.subplots()
levels = np.arange(-100000,100000,1000)
ax.contour(XJ,YJ,phii[...,0],colors='r',linestyles='-',levels=levels)
ax.contour(XJ,YJ,phii[...,1],colors='g',linestyles='-',levels=levels)
ax.set_aspect('equal')
ax.set_title('source to target')
ax.imshow(phiI.permute(1,2,0)/torch.max(phiI),extent=extentJ)
ax.invert_yaxis()
/home/kalen/.local/share/virtualenvs/STalign-VWNsoi3D/lib/python3.8/site-packages/torch/utils/_device.py:62: UserWarning: To copy construct from a tensor, it is recommended to use sourceTensor.clone().detach() or sourceTensor.clone().detach().requires_grad_(True), rather than torch.tensor(sourceTensor).
return func(*args, **kwargs)
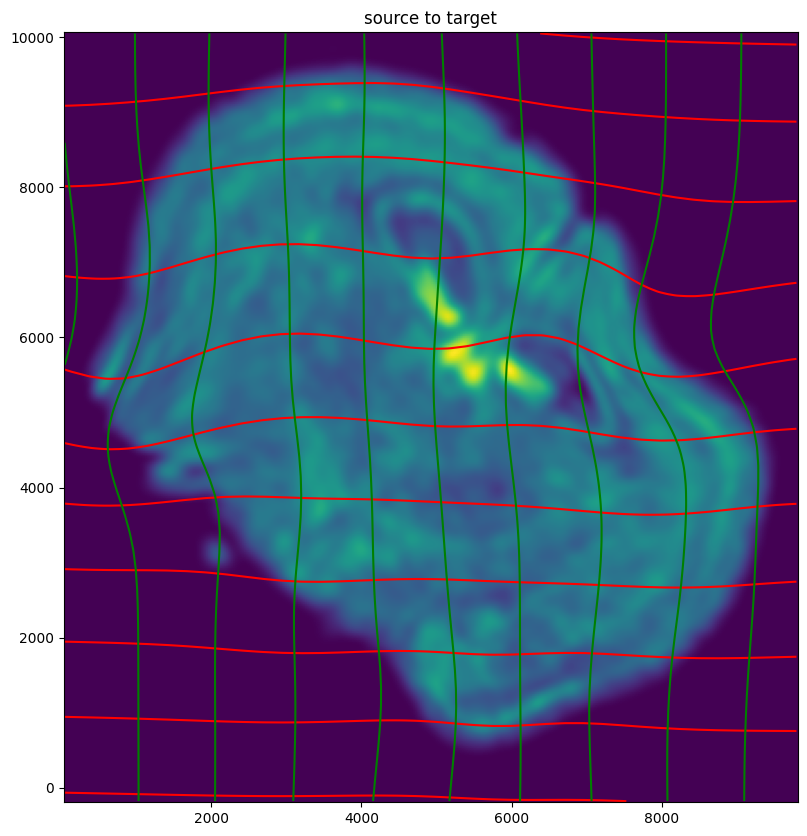
Note that because of our use of LDDMM, the resulting transformation is invertible.
[42]:
# transform is invertible
phi = STalign.build_transform(xv,v,A,XJ=[YI,XI],direction='f')
phiiJ = STalign.transform_image_target_to_source(xv,v,A,[YJ,XJ],J,[YI,XI])
#switch tensor from cuda to cpu for plotting with numpy
if phi.is_cuda:
phi = phi.cpu()
if phiiJ.is_cuda:
phiiJ = phiiJ.cpu()
# plot with grids
fig,ax = plt.subplots()
levels = np.arange(-100000,100000,1000)
ax.contour(XI,YI,phi[...,0],colors='r',linestyles='-',levels=levels)
ax.contour(XI,YI,phi[...,1],colors='g',linestyles='-',levels=levels)
ax.set_aspect('equal')
ax.set_title('target to source')
ax.imshow(phiiJ.permute(1,2,0)/torch.max(phiiJ),extent=extentI)
ax.invert_yaxis()
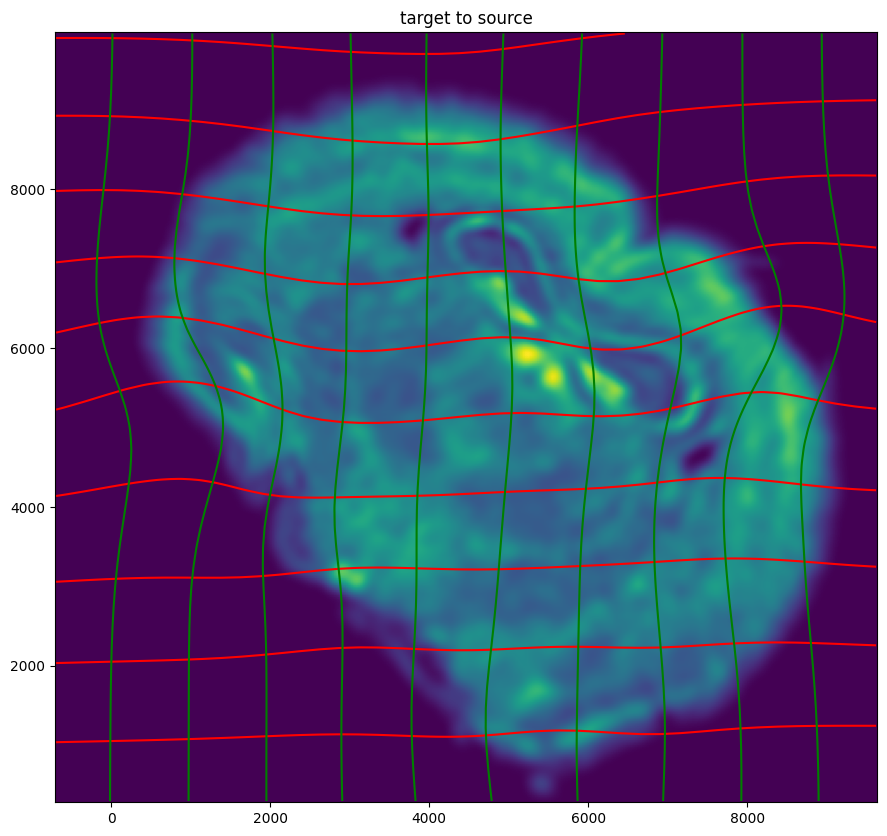
Finally, we can apply our STalign transform to the original sets of single cell centroid positions (with initial affine transformation) to achieve their new aligned positions.
[37]:
# apply transform to original points
tpointsI= STalign.transform_points_source_to_target(xv,v,A, np.stack([yI_L_T, xI_L_T], 1))
#switch tensor from cuda to cpu for plotting with numpy
if tpointsI.is_cuda:
tpointsI = tpointsI.cpu()
#switch from row column coordinates (y,x) to (x,y)
xI_LDDMM = tpointsI[:,1]
yI_LDDMM = tpointsI[:,0]
And we can visualize the results.
[38]:
# plot results
fig,ax = plt.subplots()
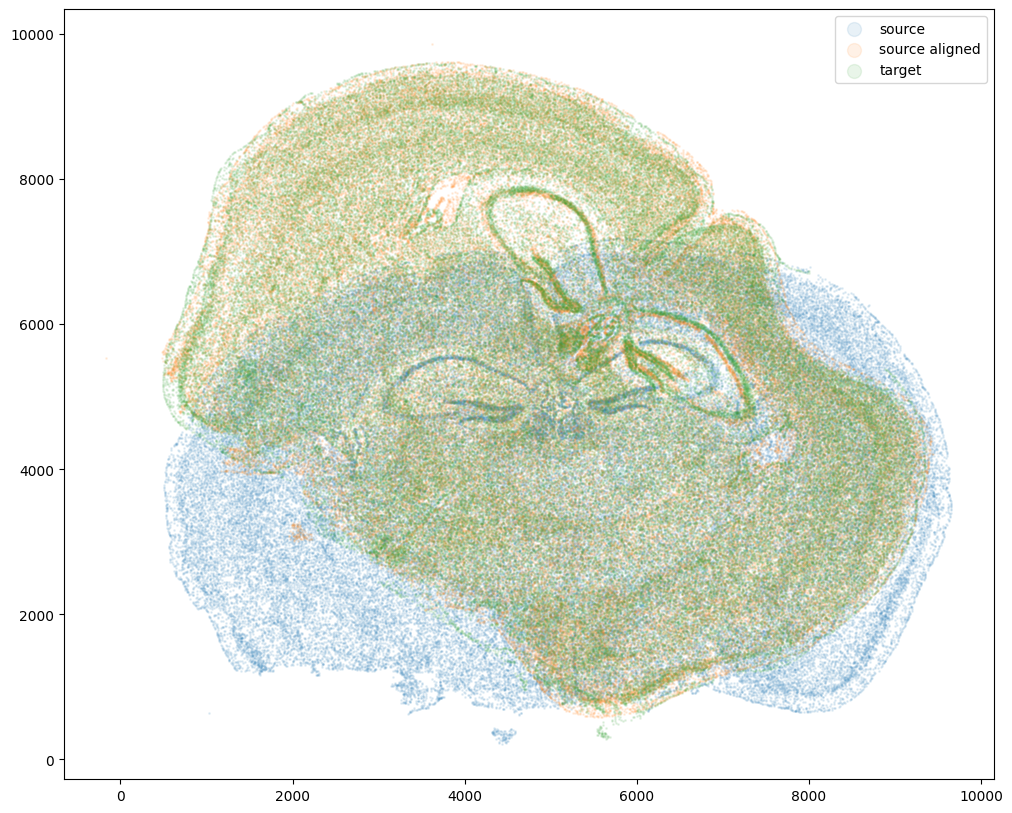
ax.scatter(xI,yI,s=1,alpha=0.1, label='source')
ax.scatter(xI_LDDMM,yI_LDDMM,s=1,alpha=0.1, label = 'source aligned')
ax.scatter(xJ,yJ,s=1,alpha=0.1, label='target')
ax.legend(markerscale = 10)
[38]:
<matplotlib.legend.Legend at 0x7fcde241de20>
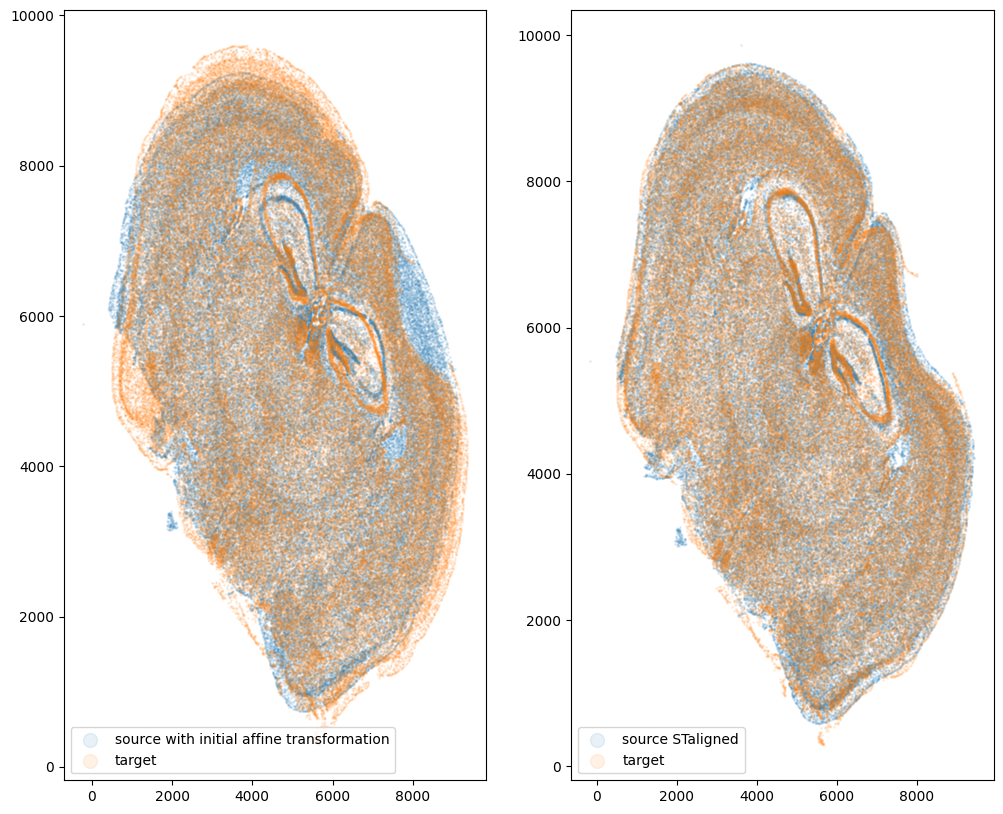
[39]:
fig,ax = plt.subplots(1,2)
ax[0].scatter(xI_L_T,yI_L_T,s=1,alpha=0.1, label='source with initial affine transformation')
ax[0].scatter(xJ,yJ,s=1,alpha=0.1, label='target')
ax[1].scatter(xI_LDDMM,yI_LDDMM,s=1,alpha=0.1, label = 'source STaligned')
ax[1].scatter(xJ,yJ,s=1,alpha=0.1, label='target')
ax[0].legend(markerscale = 10, loc = 'lower left')
ax[1].legend(markerscale = 10, loc = 'lower left')
[39]:
<matplotlib.legend.Legend at 0x7fce6a36db80>
And save the new aligned positions by appending to our original data
[40]:
df3 = pd.DataFrame(
{
"aligned_x": xI_LDDMM,
"aligned_y": yI_LDDMM,
},
)
results = pd.concat([df1, df3], axis=1)
results.head()
[40]:
| Unnamed: 0 | fov | volume | center_x | center_y | min_x | max_x | min_y | max_y | aligned_x | aligned_y | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 158338042824236264719696604356349910479 | 33 | 532.778772 | 617.916619 | 2666.520010 | 614.725219 | 621.108019 | 2657.545209 | 2675.494810 | 1072.409961 | 7618.526180 |
| 1 | 260594727341160372355976405428092853003 | 33 | 1004.430016 | 596.808018 | 2763.450012 | 589.669218 | 603.946818 | 2757.013212 | 2769.886812 | 1109.912428 | 7727.380716 |
| 2 | 307643940700812339199503248604719950662 | 33 | 1267.183208 | 578.880018 | 2748.978012 | 570.877217 | 586.882818 | 2740.489211 | 2757.466812 | 1088.557174 | 7727.036676 |
| 3 | 30863303465976316429997331474071348973 | 33 | 1403.401822 | 572.616017 | 2766.690012 | 564.937217 | 580.294818 | 2756.581212 | 2776.798812 | 1093.635775 | 7748.726637 |
| 4 | 313162718584097621688679244357302162401 | 33 | 507.949497 | 608.364018 | 2687.418010 | 603.061218 | 613.666818 | 2682.493210 | 2692.342810 | 1076.737927 | 7645.783087 |
We will finally create a compressed .csv.gz file named mouse_brain_map_BrainReceptorShowcase_Slice2_Replicate3_STalign_to_Slice2_Replicate2.csv.gz
[18]:
results.to_csv('../merfish_data/mouse_brain_map_BrainReceptorShowcase_Slice2_Replicate3_STalign_to_Slice2_Replicate2.csv.gz',
compression='gzip')