A tale of two cell populations: integrating RNA velocity information in single cell transcriptomic data visualization with VeloViz
Introduction
We recently developed a single-cell transcriptomic data visualization
method called VeloViz now published in Oxford Bioinformatics. In addition to using each cell’s observed
transcriptional state from single-cell transcriptomic data, VeloViz
also integrates information regarding each cell’s predicted future
transcriptional state inferred from RNA velocity analysis to create 2D
and 3D embeddings of the data. More details regarding how the method
works and its applications can be found in this paper in the journal
Bioinformatics. VeloViz is implemented as an R software package
avaialble at https://jef.works/veloviz/ and on
Bioconductor.
In this blog post, I will use simulations to tell a story about two cell populations, who (transcriptionally) look quite similar to each other but are going in different paths (towards divergent terminal cell fates). I hope this story will be a fun demonstration of the potential utility of integrating RNA velocity information in visualizing cell populations and their relationships.
Background and Theory
RNA velocity analysis uses the relative abundance of nascent (unspliced) and mature (spliced) mRNA to estimate the rates of gene splicing and degradation in order to predict the future transcriptional state of individual cells. In RNA velocity modeling, cells with levels of unspliced transcripts higher than expected in steady state are interpreted as having positive velocity or upregulation of the gene, while cells with levels of unspliced transcripts lower than expected at steady state are interpreted as having negative velocity or downregulation of the gene. This combination of velocities across all modeled genes can then used to estimate the future transcriptional state of each individual cell. Such RNA velocity analysis has also been adapted for transcriptomic imaging data using the relative abundance of nuclear and cytoplasmic mRNA.
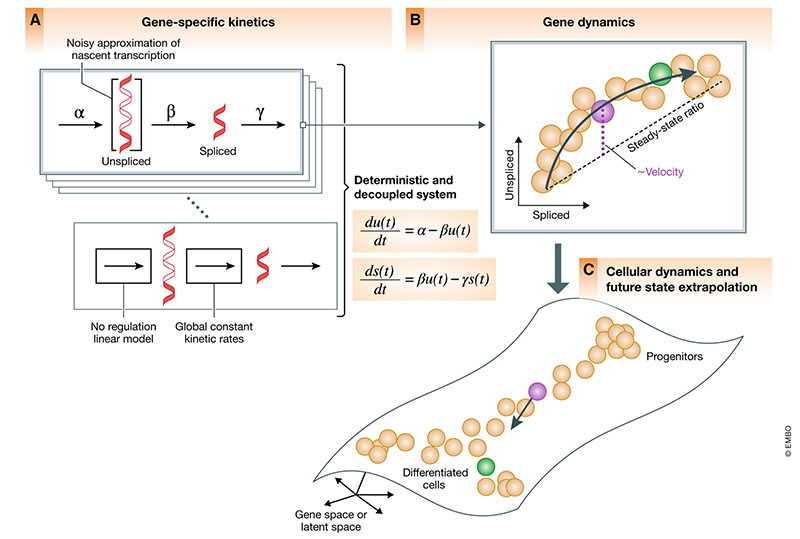
Generally, in order to visualize these predicted future transcriptional states
inferred from RNA velocity analysis, visualization with principal
components, t-distributed stochastic neighbor embedding, and other
embeddings derived from the observed transcriptional states are often
used. In the rest of this blog post, I will use a very crude simulation to
demonstrate the potential utility of integrating the predicted future
transcriptional state information from RNA velocity in deriving
embeddings for visualization purposes using VeloViz.
A tale of two cell populations (a simulation)
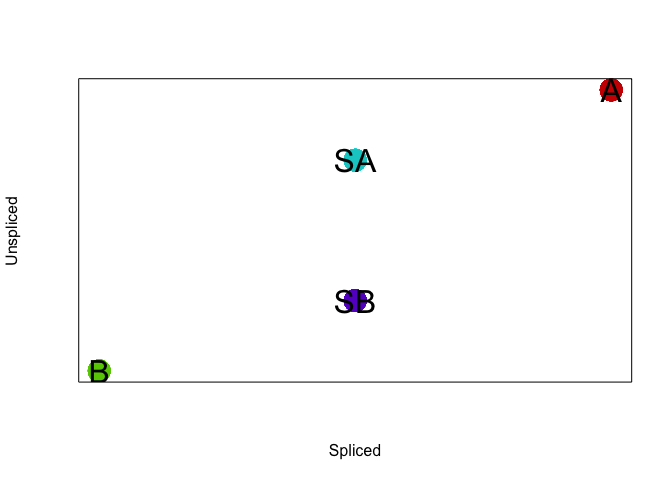
Let’s simulate some steady state cells and two intermediate cell
populations. We will simulate two steady state cell populations (A and
B) as well as two intermediate cell populations (SA and SB). In
our simulation, steady state cell populations A and B have distinct
transcriptional profiles. In contrast, intermediate cell populations
SA and SB will express genes found in both A and B. However, we will
(crudely) simulate such that SA cells are actively upregulating genes
expressed in A, while SB cells are actively upregulating genes
expressed in B following the theory behind RNA velocity. In
particular, both SA and SB will have comparable levels of spliced
RNAs but SA will have more unspliced RNAs for genes expressed in A
while SB will have more unspliced RNAs for genes expressed in B.
nc <- 100
ncss <- 50
## spliced (same)
g1s <- c(rep(10,nc), rep(10,nc))
## unspliced (one high, one low)
g1us <- c(rep(15,nc), rep(5,nc))
## simulate steady state cells
ssg1s <- c(rep(0, ncss), rep(20, ncss))
ssg1us <- c(rep(0, ncss), rep(20, ncss))
## simulated ground truth expression
gts <- c(g1s, ssg1s)
gtus <- c(g1us, ssg1us)
## group annotations
com <- c(rep('SA', nc), rep('SB', nc), rep('B', ncss), rep('A', ncss))
cell.colors <- MERINGUE:::fac2col(com)
names(gts) <- names(gtus) <- names(com) <- names(cell.colors) <- paste0('cell', 1:c(2*nc+2*ncss))
## plot
veloviz::plotEmbedding(cbind(gts, gtus), groups=com, cex=3,
xlab='Spliced', ylab='Unspliced', mark.clusters=TRUE)
## using provided groups as a factor
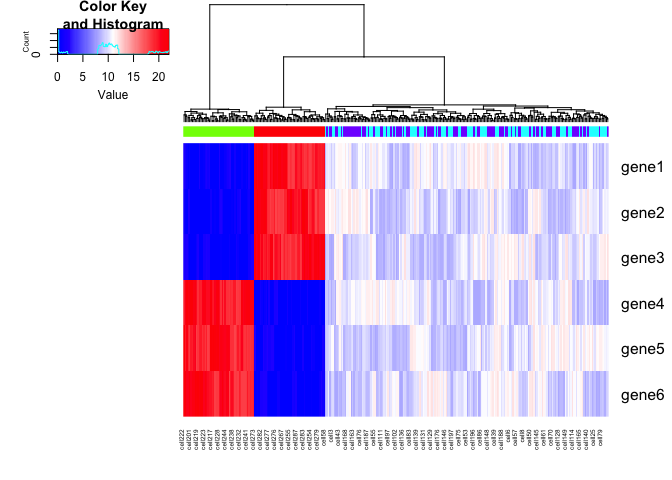
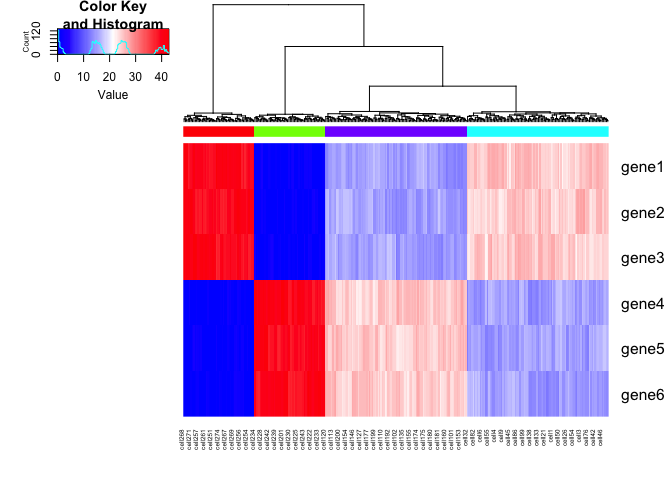
We can add some noise to the simulated data. Let’s also expand to 6
genes with the same upregulation trends as described previously. Note
that if we were to only look at the spliced gene expression levels, SA
and SB cells are transcriptionally indistinguishable. If we look at
the total gene expression levels, SA, SB, A, and B cells are be
distinct.
## add some noise
jitteramount <- 1
## make gene expression matrix with 6 genes
## where genes are upregulated in one of the intermediate populations
## to be more or less like the steady state populations (3 each)
spliced = rbind(jitter(c(g1s, ssg1s), jitteramount),
jitter(c(g1s, ssg1s), jitteramount),
jitter(c(g1s, ssg1s), jitteramount),
jitter(20-c(g1s, ssg1s), jitteramount),
jitter(20-c(g1s, ssg1s), jitteramount),
jitter(20-c(g1s, ssg1s), jitteramount))
colnames(spliced) <- paste0('cell', 1:ncol(spliced))
rownames(spliced) <- paste0('gene', 1:nrow(spliced))
unspliced = rbind(jitter(c(g1us, ssg1us), jitteramount),
jitter(c(g1us, ssg1us), jitteramount),
jitter(c(g1us, ssg1us), jitteramount),
jitter(20-c(g1us, ssg1us), jitteramount),
jitter(20-c(g1us, ssg1us), jitteramount),
jitter(20-c(g1us, ssg1us), jitteramount))
spliced[spliced < 0] <- 0
unspliced[unspliced < 0] <- 0
mat <- spliced + unspliced
colnames(unspliced) <- paste0('cell', 1:ncol(unspliced))
rownames(unspliced) <- paste0('gene', 1:nrow(unspliced))
## plot as heatmap
library(gplots)
##
## Attaching package: 'gplots'
## The following object is masked from 'package:stats':
##
## lowess
gplots::heatmap.2(spliced, Rowv=NA, trace='none', ColSideColors = cell.colors, scale='none', col = colorRampPalette(c('blue', 'white', 'red'))(100))
## Warning in gplots::heatmap.2(spliced, Rowv = NA, trace = "none", ColSideColors =
## cell.colors, : Discrepancy: Rowv is FALSE, while dendrogram is `both'. Omitting
## row dendogram.
gplots::heatmap.2(mat, Rowv=NA, trace='none', ColSideColors = cell.colors, scale='none', col = colorRampPalette(c('blue', 'white', 'red'))(100))
## Warning in gplots::heatmap.2(mat, Rowv = NA, trace = "none", ColSideColors =
## cell.colors, : Discrepancy: Rowv is FALSE, while dendrogram is `both'. Omitting
## row dendogram.
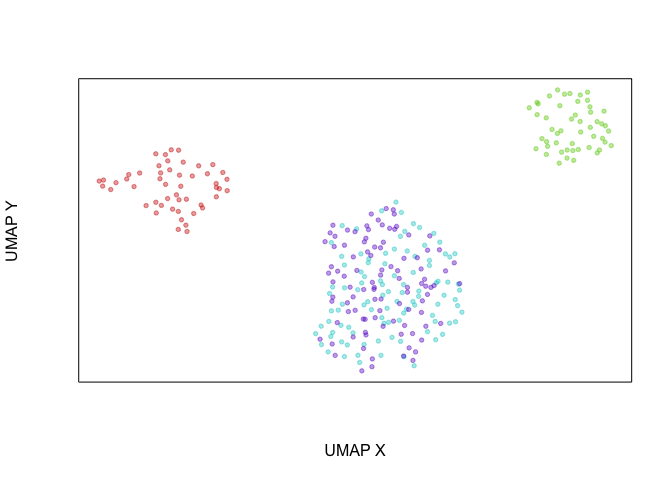
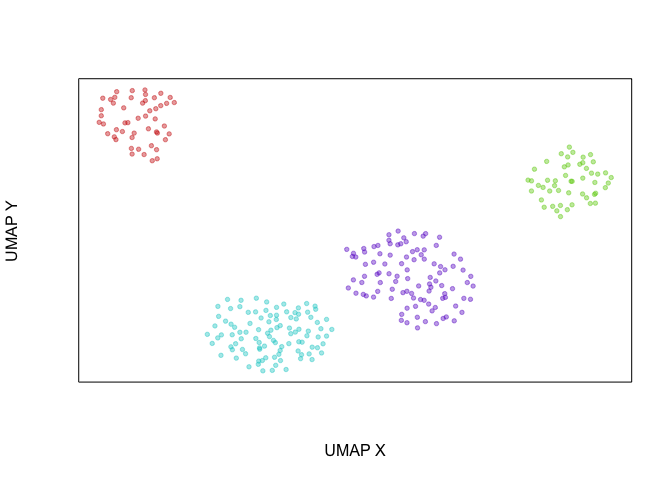
Another way of looking at this is that if we were to create an UMAP
embedding on the spliced gene expression levels, SA and SB cells
would be intermixed. Alternatively, if we were to create an UMAP
embedding on the total gene expression levels, all 4 cell populations
would form distinct clusters.
set.seed(1)
emb.umap.spliced <- uwot::umap(t(spliced), min_dist = 1, n_neighbors = 5)
rownames(emb.umap.spliced) <- colnames(spliced)
par(mfrow=c(1,1))
veloviz::plotEmbedding(emb.umap.spliced, groups=com)
## using provided groups as a factor
set.seed(1)
emb.umap <- uwot::umap(t(mat), min_dist = 1, n_neighbors = 5)
rownames(emb.umap) <- colnames(spliced)
par(mfrow=c(1,1))
veloviz::plotEmbedding(emb.umap, groups=com)
## using provided groups as a factor
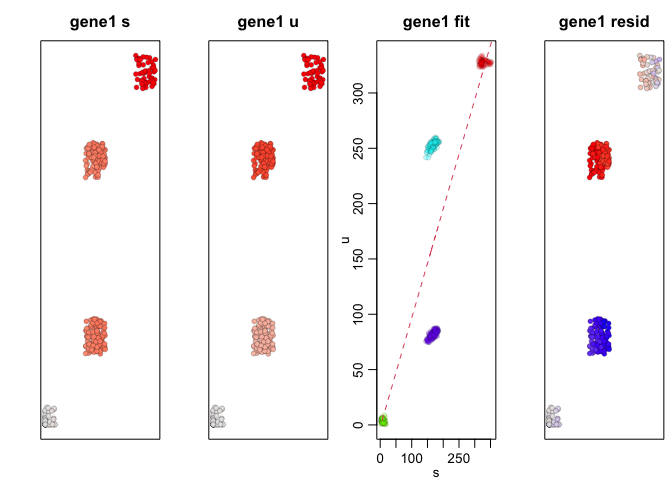
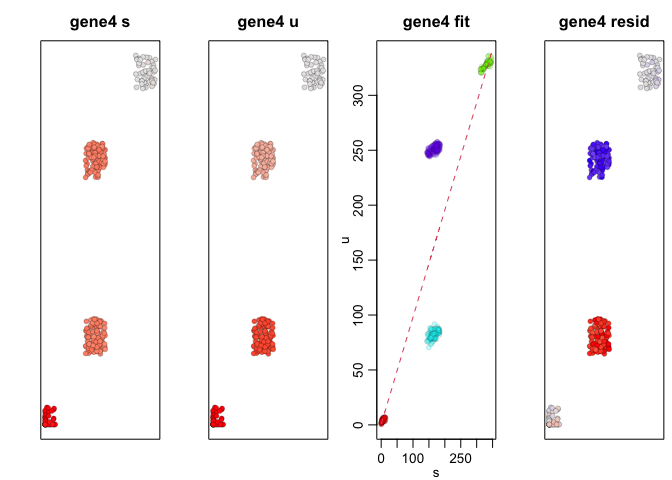
We can apply RNA velocity analysis to now predict the future
transcriptional states of our cells. Indeed, as simulated, gene1,
which is expressed in A cells but not B cells, is being upregulated
in SA cells and downregulated in SB cells while gene4, which is
expressed in B cells but not A cells, is being upregulated in SB
cells and downregulated in SA cells.
library(velocyto.R)
## Loading required package: Matrix
cell.dist = dist(t(mat))
library(velocyto.R)
vel = velocyto.R::gene.relative.velocity.estimates(spliced, unspliced, mult=1,
kCells = 10,
cell.dist = cell.dist)
## calculating cell knn ... done
## calculating convolved matrices ... done
## fitting gamma coefficients ... done. succesfful fit for 6 genes
## calculating RNA velocity shift ... done
## calculating extrapolated cell state ... done
## show gene trend
emb.test <- cbind(spliced[1,], unspliced[1,])
gene <- "gene1"
velocyto.R::gene.relative.velocity.estimates(spliced, unspliced,
deltaT=100,
kCells = 10,
kGenes=1,
fit.quantile=0.1,
cell.emb=emb.test,
cell.colors=cell.colors,
cell.dist=cell.dist,
show.gene=gene,
old.fit=vel,do.par=T)
## calculating convolved matrices ... done
## [1] 1
gene <- "gene4"
velocyto.R::gene.relative.velocity.estimates(spliced, unspliced,
deltaT=100,
kCells = 10,
kGenes=1,
fit.quantile=0.1,
cell.emb=emb.test,
cell.colors=cell.colors,
cell.dist=cell.dist,
show.gene=gene,
old.fit=vel,do.par=T)
## calculating convolved matrices ... done
## [1] 1
Therefore, at a future time point, we would expect the expression level
of gene1 in SA cells to be higher, like that in A cells, while the
expression level of gene1 in SB cells to be lower, like that in B
cells. Likewise, we would expect the expression level of gene4 in SA
cells to be lower, like that in A cells, while the expression level of
gene4 in SB cells to be higher, like that in B cells.
curr = vel$current
proj = vel$projected
par(mfrow=c(1,2))
veloviz::plotEmbedding(cbind(mat[1,], curr[1,]), groups=com,
xlab='Observed Total', ylab='Current Spliced', mark.clusters=TRUE)
## using provided groups as a factor
veloviz::plotEmbedding(cbind(mat[1,], proj[1,]), groups=com,
xlab='Observed Total', ylab='Projected Spliced', mark.clusters=TRUE)
## using provided groups as a factor
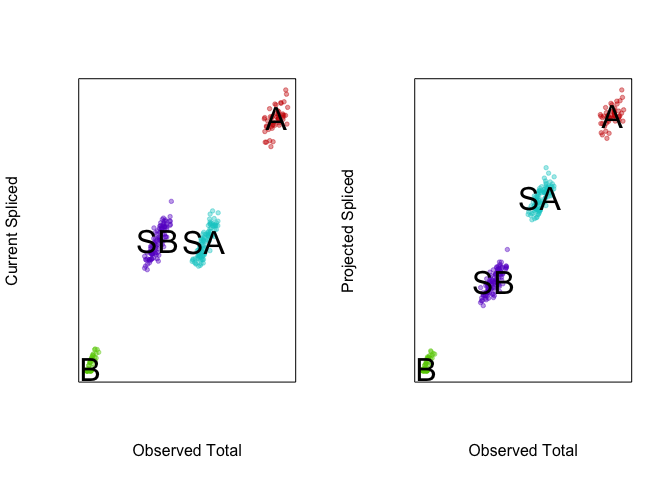
VeloViz can take into consideration this predicted future
transcriptional state in creating the embedding and visualizing these
cell populations. By taking into consideration the predicted future
transcriptional states, we can see more clearly see in the VeloViz
embedding that SA cells are closer to A cells while SB cells are
closer to B cells.
library(veloviz)
# build VeloViz graph
veloviz = veloviz::buildVeloviz(
curr = curr, proj = proj,
normalize.depth = FALSE,
use.ods.genes = FALSE,
pca = FALSE,
k = 5,
similarity.threshold = 0,
distance.weight = 1,
distance.threshold = 0,
weighted = TRUE,
seed = 0,
verbose = FALSE
)
## [1] "Done finding neighbors"
## [1] "calculating weights"
## [1] "Done making graph"
emb.veloviz = veloviz$fdg_coords
par(mfrow=c(1,1))
veloviz::plotEmbedding(emb.veloviz, groups=com, mark.clusters = TRUE)
## using provided groups as a factor
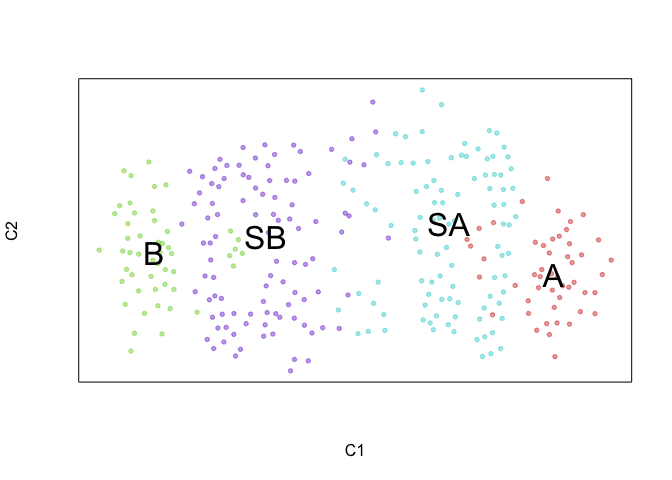
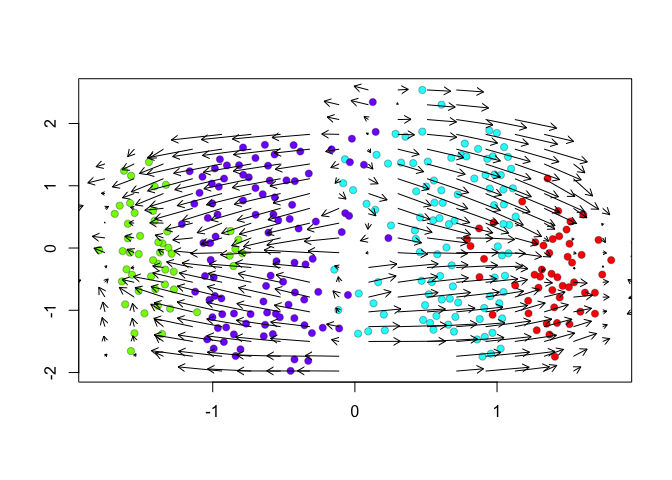
We can further visualize RNA velocity arrows on each of these embeddings to get a sense for how these 4 cell populations (SA, SB, A, and B) may be related to each other.
par(mfrow=c(1,1))
show.velocity.on.embedding.cor(scale(emb.umap.spliced), vel,n=100,scale='sqrt',cell.colors=cell.colors, arrow.scale = 0.5, arrow.lwd=1, do.par = FALSE, show.grid.flow=TRUE,min.grid.cell.mass=0.5,grid.n=20)
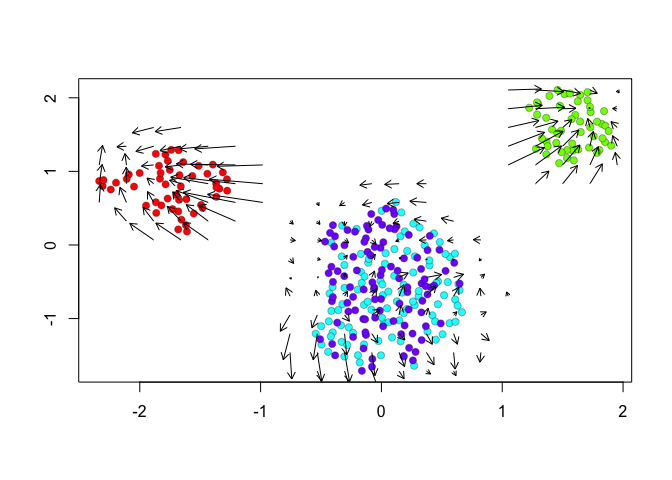
## delta projections ... sqrt knn ... transition probs ... done
## calculating arrows ... done
## grid estimates ... grid.sd= 0.1621915 min.arrow.size= 0.00324383 max.grid.arrow.length= 0.08212338 done
show.velocity.on.embedding.cor(scale(emb.umap), vel,n=100,scale='sqrt',cell.colors=cell.colors, arrow.scale = 0.5, arrow.lwd=1, do.par = FALSE, show.grid.flow=TRUE,min.grid.cell.mass=0.5,grid.n=20)
## delta projections ... sqrt knn ... transition probs ... done
## calculating arrows ... done
## grid estimates ... grid.sd= 0.1348527 min.arrow.size= 0.002697054 max.grid.arrow.length= 0.08212338 done
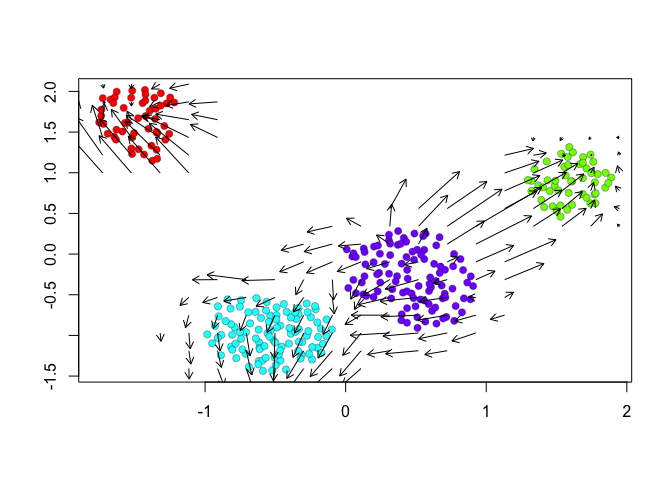
show.velocity.on.embedding.cor(scale(emb.veloviz), vel,n=100,scale='sqrt',cell.colors=cell.colors, arrow.scale = 0.5, arrow.lwd=1, do.par = FALSE, show.grid.flow=TRUE,min.grid.cell.mass=0.5,grid.n=20)
## delta projections ... sqrt knn ... transition probs ... done
## calculating arrows ... done
## grid estimates ... grid.sd= 0.146899 min.arrow.size= 0.002937981 max.grid.arrow.length= 0.08212338 done
Indeed, by taking into consideration the predicted
future transcriptional states, we can see more clearly see in the
VeloViz embedding that SA cells are transcriptionally moving towards
A cells while SB cells are transcriptionally moving towards B
cells. In contrast, UMAP embeddings that take into consideration only
the observed spliced or total gene expression levels are not able to
preserve such transcriptional relationships between populations.
Of course, this is a very crude and simple simulation highlighting the theoretical utility of taking into consideration information from RNA velocity information in visualizing cellular trajectories. Try it out for yourself! Can you simulate a scenario where taking into consideration RNA velocity information in visualizing cellular trajectories better recapitulates the underlying expected cell population relationships? What about a scenario where taking into consideration RNA velocity information misleads by suggesting a relationship between cell populations that should not exist? Are there instances with real data where these scenarios may apply? What orthogonal evidence and experiments can you do to follow up?
Additional Resources
Recent Posts
- Vibe coding with SEraster and STcompare to compare spatial transcriptomics technologies on 22 February 2026
- RNA velocity in situ infers gene expression dynamics using spatial transcriptomics data on 13 October 2025
- Analyzing ICE Arrest Data - Part 2 on 27 September 2025
- Analyzing ICE Detention Data from 2021 to 2025 on 10 July 2025
- Multi-sample Integrative Analysis of Spatial Transcriptomics Data using Sketching and Harmony in Seurat on 22 April 2025